what is sintering in ceramics
As a high-performance material, the performance of advanced ceramics depends not only on the forming process, but also on the sintering process, which is an indispensable and key step. Sintering is the process of densifying the ceramic body by heating to form a material with a specific microstructure and performance.
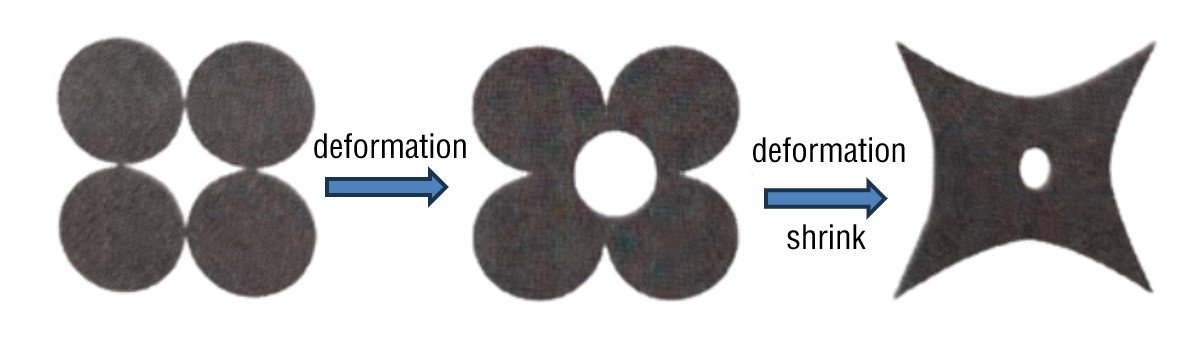
the essence of ceramic sintering
Firing and sintering of ceramics are two different concepts. The following table distinguishes and explains the two.Because the firing stage of ceramics focuses on the process of powder filling pores at high temperatures, sintering is a crucial part of the firing process. Therefore, it’s customary to loosely define firing and sintering as equivalent processes. Specifically, sintering refers to the densification process of a porous ceramic body at high temperatures, which reduces the surface area of the powder particles, lowers the porosity, and improves mechanical properties.
| Item | Firing | Sintering |
| Change | physical and chemical changes | physical changes |
| Range | Wide range, including sintering | Small range, belongs to firing |
| Definition | Dehydration, gas decomposition in the green body, multiphase reaction and melting, dissolution, formation of mineral composition, densification, and formation of microstructure. | simple physical process in which powder is densified by heat. |
Pressureless Sintering Overview
There are many sintering methods for ceramic products, and choosing the right sintering method is key to achieving the desired structure and desired properties in advanced ceramics. Pressureless Sintering (PLS) is often used in traditional ceramic production because it is performed under normal atmospheric conditions, without a special atmosphere, and offers lower production costs than specialized sintering methods. Pressureless Sintering is suitable for our most conventional ceramic materials, such as structural ceramics such as alumina (Al₂O₃) and zirconia (ZrO₂).
-
 Electric Kiln Sintering
Electric Kiln SinteringIn the traditional sintering method, heat is transferred through radiation and conduction.
-
 Ceramic Sintering Saggar
Ceramic Sintering SaggarThe carriers for sintering are usually a high-temperature resistant ceramic products.
-
 Wax Removal Before Sintering
Wax Removal Before SinteringHot-pressed advanced ceramic parts require dewaxing before sintering.
Types of Ceramic Sintering Processes
Modern advanced sintering technologies are diverse, but their core principle is to achieve material densification through different energy forms such as heat, pressure, or electric fields. The table below summarizes several mainstream advanced sintering technologies and their characteristics:
Core Principles: unidirectional high pressure to green body while heating.
Main Characteristics:lower sintering temperature, extremely high density, equipment expensive, product shapes are limited .
Application:alumina /zirconia substrates and structural components; traditional steatite and mullite ceramics
Core Principles: isotropic high temperature and high pressure (transferred through an inert gas) to the workpiece in a closed container.
Main Characteristics: isotropic and uniform density and properties, achieving near-net-shape molding; equipment is extremely expensive, production cycles are long.
Application:High-performance turbine blades, bioceramics (artificial joints), nuclear fuel and cladding materials, and critical components with extremely high reliability requirements.
Core Principles: uses pulsed direct current to rapidly heat and pressurize the mold and powder.
Main Characteristics: extremely rapid heating (up to several hundred degrees per minute) and very short sintering time (a few minutes to tens of minutes), enabling the preparation of fine-grained materials; sample sizes are typically small.
Application: laboratory research and development of new materials (such as nanoceramics, metal-ceramic composites), magnetic materials, and functionally graded.
Core Principles: uses microwaves to heat the ceramic material as a whole.
Main Characteristics: fast heating, energy efficiency, and the ability to lower sintering temperatures; however, it is technically challenging and requires specific dielectric properties of the material.
Application: alumina and zirconia electronic ceramics; and laboratory or small-batch preparation of some nitrides and carbides.
Related Process
-
precise control of raw material composition, particle size and uniformity.
-
make blanks (greenware) with a certain shape and size from prepared ceramic raw materials.
-
perform high-precision and low-damage surface treatment on sintered ceramic parts.
-
-
get a thin layer of metal onto a ceramic surface, for ceramic-metal connecting .
Ceramic sintering – important ceramic manufacturing process
During the sintering process, the ceramic body undergoes a series of physical and chemical changes, including expansion, contraction, gas generation, liquid phase formation, disappearance of old phases, and formation of new phases. The quality of ceramic sintering is often reflected by physical indicators such as sintering shrinkage, strength, apparent density, and porosity.
Different ceramic materials, temperatures, atmospheres, and pressures will produce different changes, which determine the quality and performance of ceramic products.
Time and cost
The time required for ceramic sintering accounts for 1/4 to 1/3 of the entire production cycle of ceramic products, and the required cost accounts for about 40% of the total product cost.
Product quality assurance
Correctly designing and selecting firing kilns, scientifically formulating sintering process parameters and technical documents, scientifically and rationally stacking batches of ceramic blanks, and strictly implementing sintering operation specifications are necessary guarantees for improving product quality and reducing fuel consumption.
The following four factors can be considered comprehensively:
1. Pursuit of ultimate speed and energy saving: Microwave sintering is worth considering.
2. Need for customized parts with complex shapes: Laser sintering can be considered.
3. Sintering ultra-high temperatures or special materials: Plasma sintering or induction heating hot pressing are the directions.
4. Balancing performance and industrial mass production: Traditional electric kilns and microwave sintering are the mainstream.
Increasing the sintering temperature facilitates solid-phase diffusion, but simply increasing the sintering temperature wastes fuel and is uneconomical. Furthermore, excessively high sintering temperatures can lead to secondary recrystallization, deteriorating product performance. In sintering with a liquid phase, excessively high temperatures increase the liquid phase, decrease viscosity, and cause product deformation. Therefore, the sintering temperature must be properly controlled.
Both melting and sintering are caused by atomic thermal vibrations, but in melting all components are transformed into liquid phase, while in sintering at least one component is in solid phase. Sintering is carried out at a temperature far below the melting temperature of the main components.








